Micron crystal "flower"
Inspired by the biomineralization of nature, scientists have obtained micron-scale, crystal-like crystals in the laboratory. Learning from the formation of diatoms and abalone shells in nature, crystal growth researcher Harvard University (Wim Noorduin) and his team improved the method of crystal growth in the laboratory by changing the temperature of the solution. pH and carbon dioxide concentration, to achieve crystal growth process controllable. All the complexity stems from the simplicity. The interaction between inorganic minerals and organic molecules in the natural world can generate highly functional, structured materials. The “vase, stem, leaf and flower†of the crystals in the laboratory is also born. Inspiration from nature “The original idea of ​​Crystal Rose came from a book I was reading at the time, Philip Ball's Pattern Formation in Nature,†Noduyne told Petra. This book describes how patterns can be generated in natural and artificial systems and how sensitive environments control pattern generation.The process of pattern formation in the book led me to start thinking about whether there is such a system that can freeze patterns into solid state And the pattern remains controllable during growth?†“It is a very interesting experience to swim and indulge in the microcosm created by myself!†Noduyne told the Nutshell Web. “Initially, I mainly wanted to find ways to control the formation of different microscopic shapes. However, when I learned After building a library of different shapes and patterns, I was completely addicted to building more complex and beautiful shapes." How to make a crystal flower How can I make a beautiful crystal flower? The raw material you need is just a beaker of an aqueous solution of barium chloride and sodium silicate. Put the "flower garden" - a flat piece of liquid into the solution, fill with carbon dioxide, and carefully adjust the reaction temperature, your little flower can grow on the "flower garden". When the pH of the solution changes from 8 to 12, the precipitation of silica and cesium carbonate solids changes as follows: All crystal growth begins with nucleation of cesium carbonate, which reduces the pH. Starting from a high pH solution and continuously injecting carbon dioxide, the following reactions occur: Ba2+ + CO2 + H2O → BaCO3 + 2H+ The released hydrogen ions continuously reduce the pH at the front end of the growth until it enters the pH range where silica crystals can precipitate: SiO32–+2H+→SiO2+H2O Therefore, Stage 1 achieves the coprecipitation of cesium carbonate and silica. In this stage, the cesium carbonate crystal grows best when the bulk solution pH is high; at low pH, the crystal growth is inhibited by silica precipitation. According to the different nucleation density, three different ground state shapes are generated. The hemispheres are formed where the nucleation density is low, and stem shapes and cones are formed where the nucleation density is high. The diffusion zone around the crystal at high pH controls its shape. With the addition of CO2, the pH of the bulk solution gradually decreased. When the solution is lower than the optimal pH for the formation of silica, but still higher than the pH required for the precipitation of SiO2 (pHSiO2), injecting a bulk solution with a pH greater than pHSiO2 promotes the formation of silica and inhibits the growth of barium carbonate crystals. In order to maintain the low pH of the growth front, the barium carbonate crystals in Phase 2 tend to grow along the interface or curl. Finally, when the pH is lower than pHSiO2 (stage 3), the silica gradually stops precipitating and the barium carbonate crystals can grow normally. Under the electron microscope Observations by scanning electron microscopy (SEM) showed that the "petals" of the crystal flower were 1 μm thick. The researchers found that the injection of carbon dioxide can increase the amount of barium carbonate precipitated, so that during the crystal growth process, the thickness of the "petal" can be adjusted by the increase and decrease of carbon dioxide. In addition, lowering the temperature has the same effect as increasing carbon dioxide. The thickness of the crystal can also be adjusted by the concentration of salt in the solution (for example, increasing sodium chloride can promote silica precipitation) without affecting the structure of the original cerium carbonate crystal. Knowing the chemical mechanisms behind "flowering" and "crimping", researchers can change the parameters to get the desired structure. The seemingly simple superposition of mechanisms and patterns can create an exquisite microscopic world. However, the birth of a crystal rose has been devastating. "From the beginning of the flash of light to the final publication of the paper, I spent more than three years with his colleagues and experienced more than 1,000 experiments." Noduin told the shell network, "Although we soon have There are theoretical assumptions, but it takes years to determine the details of these mechanisms and whether they can fully control the experiment." Noordyne recalled that a huge challenge at that time was that these chemical reactions were very sensitive to changes in conditions. After adjusting the experimental conditions, it was often difficult to figure out exactly what happened. "For example, just the simple act of opening a fume hood may have drastically altered the crystal structure," said Noduyne. "Given that crystals are highly sensitive to experimental conditions, it took me a long time to learn to control these reactions. And use this sensitivity to shape these structures.†Another challenge is to perfect a large number of techniques for "growing" this microstructure. “When I started to build these layers, I had to optimize the structure of the crystals so that these structures would not be destroyed or dumped during the experiment.†Geek romance “Writing information in rhythmic patterns, cultivating a tulip-like structure, or creating a 'flower stem' in a 'vase' to make a bunch of flowers, I would like to explore all possibilities because you can plant thousands of them at the same time. The flowers really feel a bit like swimming on exotic coral reefs." Noordyne said that in the past few years he has produced and photographed thousands of photos of crystal roses. "I will pick some best looking pictures, color the structure in the picture, and then give them to my girlfriend," he said. It is conceivable that Noduyne's girlfriend should have been pleasantly surprised to receive photos of these microscopic flowers. make persistent efforts A year ago, with this amazing skill, Noduyne and his colleagues published their papers in Science. Currently, Noordyne and his colleagues are working hard to develop a mathematical model that simulates the growth of crystals and reveals some details in the crystal growth process. “Based on our understanding of the crystal growth mechanism, we can certainly obtain the structure we want by adjusting the experimental conditions. To be able to obtain complex structures that can be generated in multiple steps and can be controlled continuously, we must optimize every single one of the experiments. The steps.†Noduyne pointed out that in order to make a structure that can be formed through multiple steps, they have to perfect the operation of each step. “The extent to which we can do so is not yet certain. We are still exploring how much more refined shapes can be made using this method, and we are also exploring new ways to create more complex structures,†he said. Although Novartis used calcium carbonate and strontium carbonate as the model system in the experiment, the above mentioned design strategy can also be applied to other compounds. Nouduyne's team is broadening the types of materials that can be used and is developing technologies that can better control the shape of crystals. Nouduin said to the shell network: "This increasingly sophisticated microstructure construction technology may be close to the requirements of many practical applications for conditional control, such as the preparation of optical materials, catalysts, etc." Robot Welding,Sheet Metal Welding,Welding Fabrication Custom,Metal Welding Custom JIANGSU TONGDE INTERNATIONAL TRADE CO.LTD. , https://www.jstongdetrade.com
Noduin got the first crystal flower. 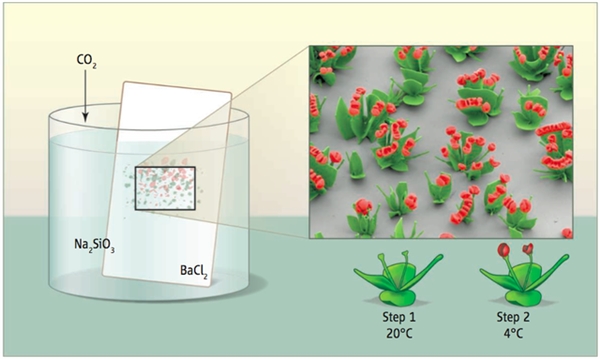
Crystal flower preparation schematic. 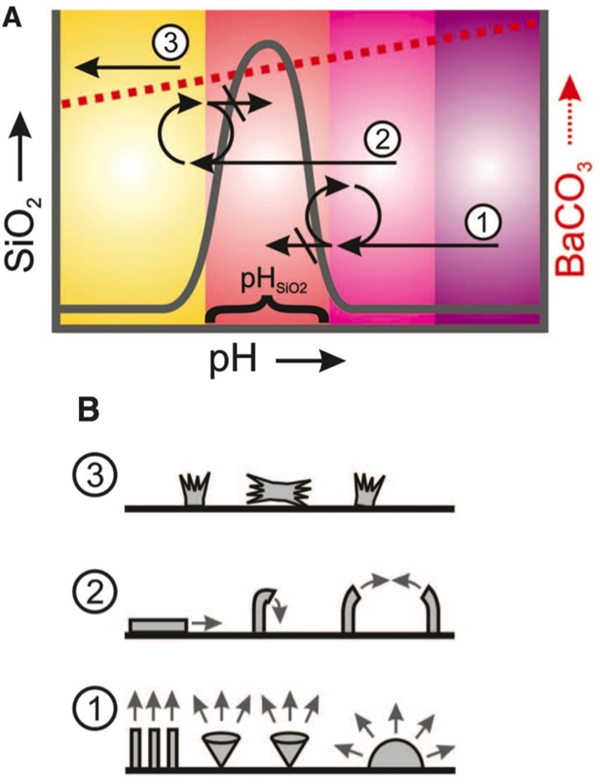
Controllable Crystal Growth Mechanism and Three Basic Crystallization Patterns