Institute of Physics and other Institute of room-temperature sodium ion tunnel oxide electrode material research won
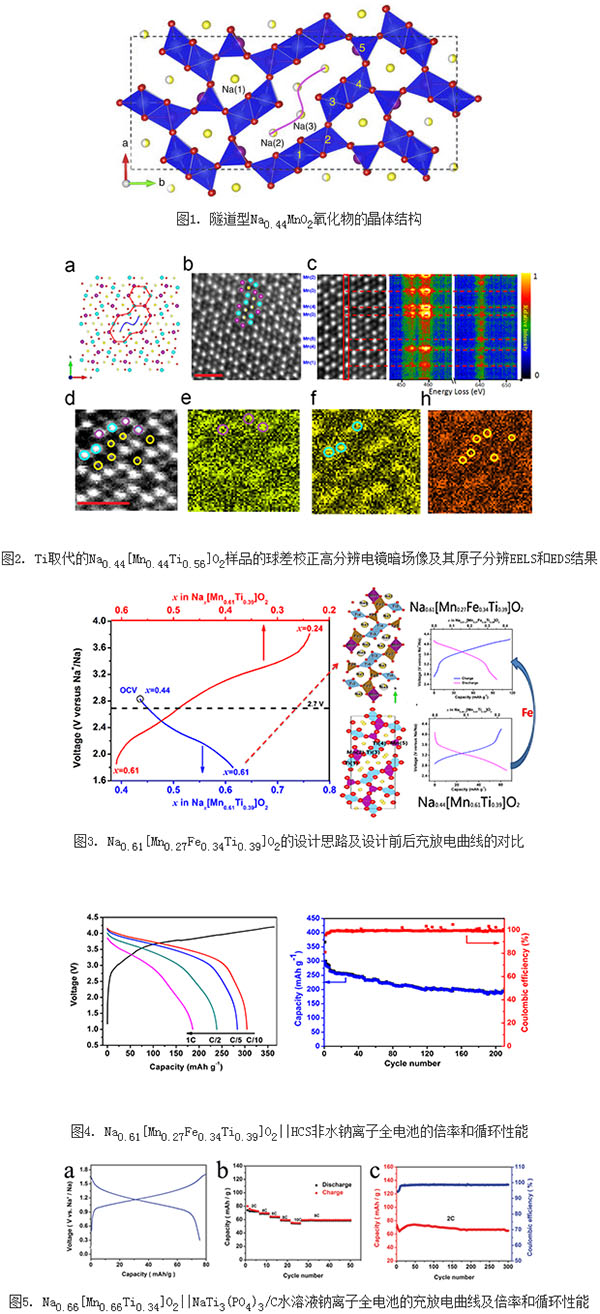
Since sodium is abundant in the earth's crust and widely distributed, sodium has similar physical and chemical properties to lithium, and storage mechanisms, it is of great strategic importance to develop room-temperature sodium ion batteries for large-scale energy storage applications. Currently, the electrode materials studied mainly include layered oxides, tunnel oxides, and polyanionic compounds. Relative to oxides, the synthesis step of polyanionic compounds is generally more complex and requires carbon coating to increase its conductivity. The layered oxides have stability problems due to water absorption or reaction with water-oxygen/water-carbon dioxide, and cannot be stored in the air for a long period of time; and there are many phase transitions in the electrochemical cycle, and the structure changes greatly. Long-term cycle stability. The tunnel-type oxide Na0.44MnO2 has a unique S-type channel (Fig. 1), which ensures structural stability during cycling and is very stable in air and water. However, the disadvantage is that the charge capacity in the first week is only half of the reversible capacity, that is, the actual usable capacity is only about 50 mAh/g. Physician Wang Yuesheng of the Institute of Physics of the Chinese Academy of Sciences/Beijing National Laboratory for Condensed Matter Physics, under the guidance of Hu Yongsheng, a researcher at the E01 group of clean energy laboratories, systematically studied Ti-substituted Na0.44MnO2 and A04. Group researchers Gu Lin, Professor Yang Xiaoqing of the Brookhaven National Laboratory, Dr. Yan Xiqian, Professor of National University of Seoul, Kisuk Kang, South Korea, and Dr. Yang Wanli of Lawrence Berkeley National Laboratory, etc., adopted high-resolution spherical electron microscope technology, synchrotron radiation technology, and The first-principles calculations accurately determine the positions occupied by Mn and Ti in the structure and the transition metals responsible for charge compensation during electrochemical processes and their locations (Figure 2). The replacement of Ti breaks the original Mn4+/Mn3+ charge ordering, further affecting the reaction path, thereby smoothing the charge-discharge curve and reducing the discharge voltage. In addition, Ti-substituted samples can be used as negative electrode materials for aqueous sodium ion batteries, exhibiting excellent cycling performance without deoxygenation. The results of the relevant studies are published in Nature Communications 2015, 6: 6401. After understanding the position and valence of each transition metal in the tunnel structure and the charge compensation mechanism, doctoral student Xu Shuyin and researcher Hu Yongsheng proposed a positive electrode material design method (as shown in Figure 3) that will have a high potential. The Fe4+/Fe3+ redox couple was introduced into the Na0.61[Mn0.61Ti0.39]O2 discharge state of the Ti-substituted sample, and a stable tunnel-shaped oxide cathode with high sodium content and high Fe content was designed. Material Na0.61 [Mn0.27Fe0.34Ti0.39]O2. Gu Lin determined the position of each atom in the material and its structural changes during charging and discharging by high-resolution spherical aberration correction electron microscopy. The cathode material in the voltage range of 2.5-4.2 V, the first week of its reversible capacity of up to 90mAh / g, while showing a higher discharge voltage (3.56 V); M04 group researcher Yang Haitao and researcher Cheng Zhaohua using Musburgh Ernest's spectrum confirms that the Fe4+/Fe3+ redox couple participates in the electrochemical reaction during the charge-discharge process. This is the first time that a reversible transition of the Fe4+/Fe3+ redox couple has been realized in the tunnel oxide. The energy density of a non-aqueous sodium ion full battery assembled using the positive electrode and the hard carbon negative electrode can reach 224 Wh/kg (calculated based on the sum of the positive and negative electrode masses), showing a good rate and cycle performance (FIG. 4). More importantly, the elements Na, Fe, Mn, and Ti used in this material are abundant in the earth's crust and environmentally friendly, and are suitable for the development of large-scale sodium-ion batteries for energy storage. In addition, doctoral student Wang Yuesheng and researcher Hu Yongsheng also designed a relatively low voltage tunnel oxide cathode material Na0.66[Mn0.66Ti0.34]O2, which can be used as a cathode material for aqueous sodium ion batteries, and coated with carbon. The NaTi3 (PO4) 3/C anode material assembled an aqueous sodium ion full battery with an average output voltage of approximately 1.2 V, demonstrating excellent rate and cycling performance (Figure 5). The relevant research results were published in Nat. Commun. 2015, 6: 6401, Adv. Energy Mater. 2015, 5, 1501156 and Adv. Energy Mater. 2015, 5, 1501005. This series of work was supported by the National Natural Science Foundation of China's Outstanding Youth Fund, the "863" Innovation Team Project of the Ministry of Science and Technology, the Innovation Group of the Committee and the 100-person Plan of the Chinese Academy of Sciences. Solar Street Light,Outdoor Solar Street Lights,Solar Light,Solar Street Lamp China Searun Solar Solution Co., Ltd. , https://www.srsolarlights.com